
A revolutionary field test for East Coast fever
Scientists at the International Livestock Research Institute have developed a highly sensitive, affordable and rapid test for East Coast fever (ECF).
The test breaks new ground by using a variant of the Cas enzyme, popularized for its use in CRISPR gene editing and has been specifically tailored for farmers to carry out in field conditions at the pen-side of livestock holdings.
And the technology poses more exciting possibilities.
“The beauty of it is you can adapt this technology to any disease,” says Nicholas Svitek, ILRI senior scientist and PhD supervisor of Robert Muriuki, the two researchers who developed the test.
Why farmers struggle with ECF
ECF is a pervasive disease of cattle which is caused by the Theileria parva parasite, a single-celled microorganism small enough to live inside cattle white blood cells. T. parva is spread by another, larger parasite, the tick, which sucks up and injects sporozoites (the infecting stage of the parasite) in cattle when feeding.
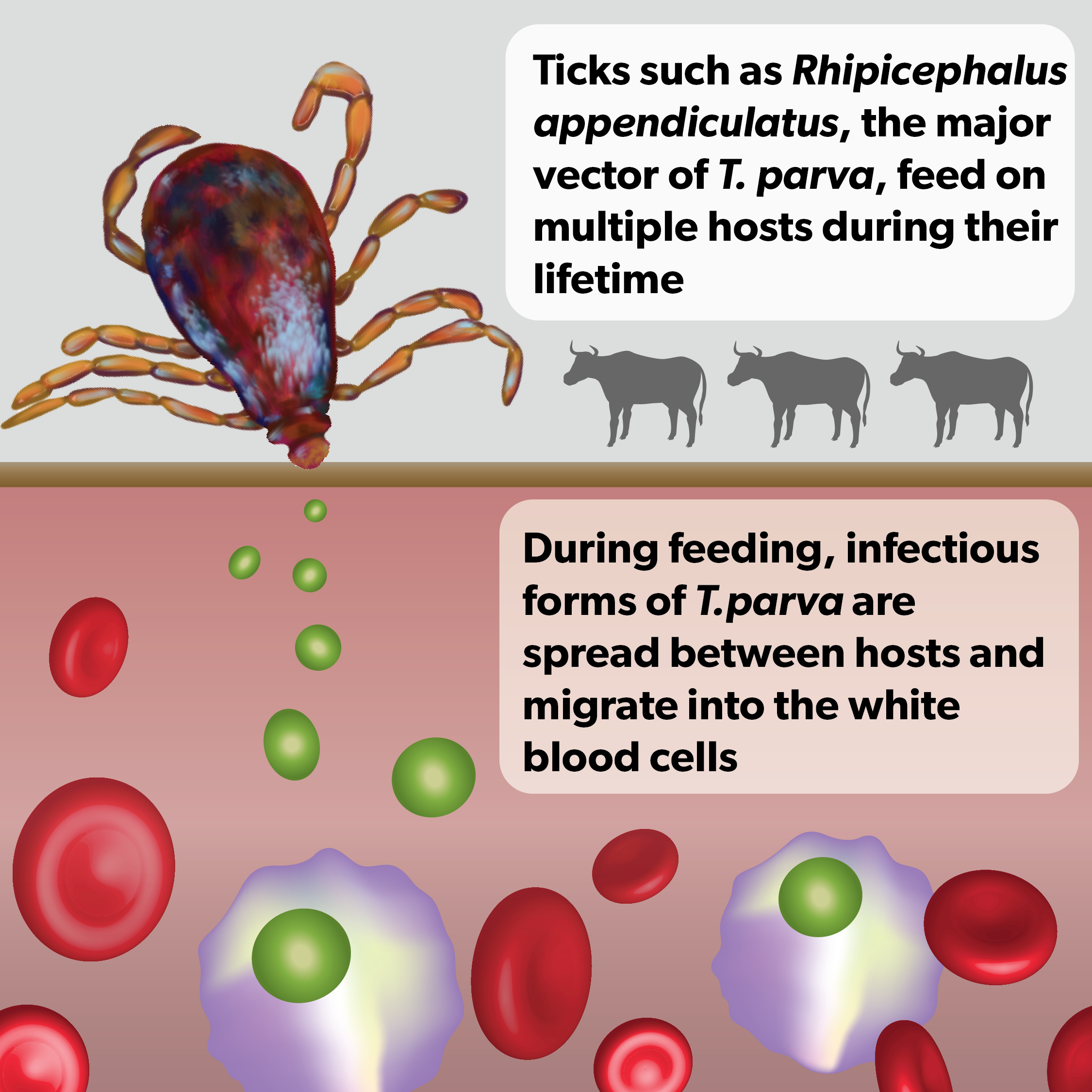
The disease is a major constraint on cattle farming. Endemic in over a dozen African countries, its symptoms can range from mild to severe in cattle, causing fever, difficulty breathing and muscle wasting. Cattle may die within three weeks after infection, and any that survive will remain weaker and less productive.
There are drugs to treat ECF, and there is a vaccine. But these options are often limited by costs and availability. If a farmer notices an animal appearing sick, they need to know the best course of action. What treatment drugs should they purchase? If it looks like an infectious disease, are other animals likely to have been affected, and which should be vaccinated?
Furthermore, as ECF can be so prevalent in some areas, farmers will often assume it is the cause of symptoms and provide treatment accordingly. But if the cause is actually another disease, they will still end up losing an animal.
In short, what farmers need is a reliable, sensitive and fast test for ECF that can be used in the field. This has not existed—until now.

The new approach
The current method to diagnose ECF relies on taking a blood sample, then transporting it to a specialized laboratory to analyze it for traces of T. parva DNA. This process can take weeks, and in the meantime the disease might continue to spread within herds or across land regions as cattle are moved. Animals might die, and a farmer and family’s livelihood may be ruined.
Svitek and colleagues thought of another approach in 2021. Since its first publication in 2012, the Nobel Prize-winning technology of CRISPR-Cas9 gene editing has been making a growing wave in the field of molecular biology. The approach uses specialized DNA components found in bacteria—the so-called CRISPR section can identify specific DNA sequences, while the Cas9 enzyme can cut them out.
But there are other variants of Cas enzymes, and one is Cas12a. Once a CRISPR-Cas12a complex identifies a specific sequence of DNA, it cuts it out… but like a runaway machine, the complex then continues cutting apart any nearby DNA.
In effect, this means the presence of a specific target DNA code causes the enzyme to produce a confetti-like cloud of DNA shreds. This isn’t helpful for precise genome editing, but this effect could be invaluable for developing sensitive diagnostic disease tests. Because the ideal diagnostic test should react to the tiniest trigger—in this case, a piece of DNA from a disease-causing microorganism—and signal this through a rapid, obvious response.
As the research community began exploring this potential, Svitek and colleagues wrote a proposal to the United States Agency for International Development Feed the Future Animal Innovation Lab for funding to develop an ECF test using Cas12a.
Muriuki, a PhD student at ILRI and the University of Nairobi, already had experience in developing field diagnostic tests and researching ECF. He ended up developing the prototype test as his PhD project, beginning in 2021.
Muriuki engineered a CRISPR-Cas12a/guide RNA complex to seek a DNA sequence specific to T. parva. He also added a molecule that gives a signal if the sample is positive—in this case, a DNA probe that emits fluorescence—to the complex. If the T. parva DNA was found and the runaway cutting triggered, the fluorescence extremity would be destroyed. This means that a test showing absence of fluorescence was present would also indicate whether T. parva is present.
For the test, the scientists devised paper-like strips, similar to those used in lateral flow COVID-19 tests, which make the fluorescence measurable by accumulation of a signal at a test line. As there are multiple strains of T. parva, Muriuki also proved the test could specifically identify eight different ECF-causing T. parva strains, but would not be triggered by other Theileria species that did not cause the disease.

When carrying out all steps including DNA extraction from cattle blood samples, the new test can give results in just 1.5 hours, at unprecedented sensitivity. It can detect the presence of a single T. parva-infected cell within 3 microlitres of blood containing several thousands of blood cells. This means the test could diagnose ECF infection even before clinical symptoms manifest.
Putting it in the field
Muriuki and Svitek designed the test keeping in mind the simple equipment and conditions that would be typical for rural veterinary officers or farmers. And affordability was another major goal. Currently the test costs an estimated KES 1000 (USD 7.78). But if key components are made in-house rather than being bought and imported, they believe the cost could be reduced to less than KES 200 (USD 1.55).
The researchers carried out a two-day workshop in Narok county with veterinary officers and farmers, showing people how to extract testable DNA from blood samples, and how to apply this to the ECF test.

The vets were happy with the test.
“But I think what made our day was how the farmers responded,” Muriuki remembered.
Farmers were excited at the prospect of being able to diagnose their animals easily and said they would be willing to buy the test first, at the current estimated price of USD 7.78, to see if ECF treatment was necessary. The farmers also gave the researchers feedback on how to simplify the steps of testing, and more invaluable ideas.
“They loved it, but they were hoping we could also put in a way that could not only detect ECF, but other tick-borne diseases,” said Muriuki.
This is because many animals can have co-infections of diseases.
The future
At the time of the field visit, the researchers had only developed the T. parva test. But they say they have prototype tests for two other parasitic cattle diseases—babesiosis and anaplasmosis. And they believe they could combine at least two, maybe three diagnostics into one single test.
It took somewhere up to two and a half years to create this test for ECF. But now with Muriuki’s expertise in identifying suitable DNA sequences and optimizing the CRISPR-Cas12a assay, they have already developed additional prototype tests in just six months.
Using CRISPR-Cas12a in disease diagnostics is cutting-edge technology, with no commercial tests yet available. But this is only a matter of time. The scientists are optimistic about the future of the test for the benefit of governments, communities, and individual farmers, if they can obtain more funding to make it even simpler for use, more affordable, and capable of diagnosing more diseases.
“At the end of the day, the whole idea behind this diagnostic project was basically to have impact for the farmer,” Svitek reflects. “And we feel we’ve achieved much of that, and now need to focus on bringing it to market.”
“It shows the potential that we as an institute have, to deal with such diseases,” says Muriuki.
To him, the possibilities are endless.
The initial phase of the project was dedicated to designing and developing the test. With the first prototype now available, the researchers plan to engage with the private sector to explore how to provide the test locally, and how to develop a multiple diagnostic test. Before moving onto this second phase in 2025, they will complete additional validation with more clinical samples before the end of this year.

Further reading:
The challenges surrounding ECF are explained in an episode of ILRI’s podcast, The Boma, by Vish Nene, former co-lead of the Animal and Human Health program at ILRI.


















